The purpose of this study was to develop and validate an LC-MS/MS method for assaying N-acetylmannosamine (ManNAc) in human plasma (K2EDTA) to support a phase I clinical study.
GNE myopathy, previously known as hereditary inclusion body myopathy (HIBM) is an autosomal recessive, muscular disorder, characterized by progressive muscle weakness with onset in early adulthood. ManNAc is being investigated by NIH and New Zealand Pharmaceuticals, as the precursor of sialic acid, prevents the development of muscle disease in the mouse model of GNE myopathy.
As an endogenous compound, calibration standards for analyzing ManNAc in human plasma need to be prepared in surrogate matrix. The presence of multiple hydroxyl groups makes it difficult to use reversed-phase chromatography due to the lack of retention. Alliance Pharma developed a HILIC LC-MS/MS method with a calibration range of 10 to 5000 ng/mL. The method has been validated thoroughly to support clinical trials of ManNAc in GNE myopathy patients. The research was funded by NCATS via NCI Contract No. HHSN261200800001E.
Method
ManNAc has four hydroxyl groups and relatively low molecular weight (221 amu). Its chromatography in various reverse phase columns had poor peak shape and low sensitivity. This problem was addressed by using an amide column in HILIC mode with trifluoroacetic acid (TFA) as the mobile phase modifier. The matrix background caused severe ion suppression which was probably due to the phospholipids in acetonitrile crashed human plasma. The suppression problem was solved by utilizing a phospholipid removal plate in the extraction step. Fifty microliters of human plasma was extracted with ManNAc-13C-d3 as the internal standard. No matrix effect was observed by comparing the results from QCs prepared in matrix and prepared in neat solution.
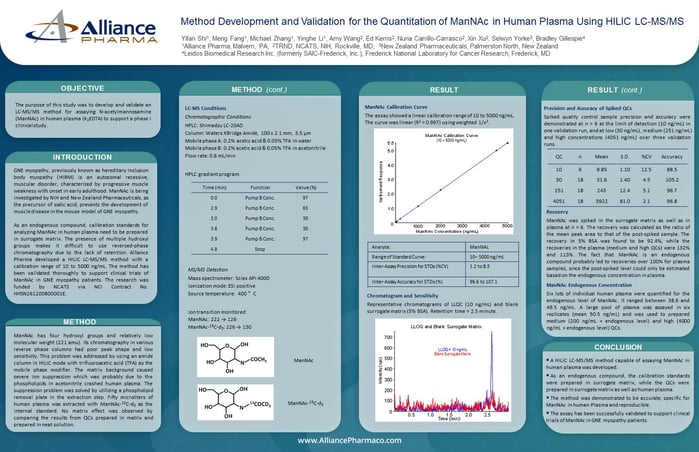
LC-MS Conditions
Chromatographic Conditions
HPLC: Shimadzu LC-20AD
Column: Waters XBridge Amide, 100 x 2.1 mm, 3.5 µm
Mobile phase A: 0.2% acetic acid & 0.05% TFA in water
Mobile phase B: 0.2% acetic acid & 0.05% TFA in acetonitrile
Flow rate: 0.8 mL/min
MS/MS Detection
Mass spectrometer: Sciex API 4000
Ionization mode: ESI positive
Source temperature: 400 °C
Ion transition monitored:
ManNAc: 222 → 126
ManNAc-13C-d3: 226 → 130
Results
ManNAc Calibration Curve
The assay showed a linear calibration range of 10 to 5000 ng/mL. The curve was linear (R2 > 0.997) using weighted 1/x2.
Chromatogram and Sensitivity
Representative chromatograms of LLQC (10 ng/mL) and blank surrogate matrix (5% BSA). Retention time = 2.5 minute.
Precision and Accuracy of Spiked QCs
Spiked quality control sample precision and accuracy were demonstrated at n = 6 at the limit of detection (10 ng/mL) in one validation run, and at low (30 ng/mL), medium (251 ng/mL) and high concentrations (4051 ng/mL) over three validation runs.
Recovery
ManNAc was spiked in the surrogate matrix as well as in plasma at n = 6. The recovery was calculated as the ratio of the mean peak area to that of the post-spiked sample. The recovery in 5% BSA was found to be 92.4%, while the recoveries in the plasma (medium and high QCs) were 132% and 113%. The fact that ManNAc is an endogenous compound probably led to recoveries over 100% for plasma samples, since the post-spiked level could only be estimated based on the endogenous concentration in plasma.
ManNAc Endogenous Concentration
Six lots of individual human plasma were quantified for the endogenous level of ManNAc. It ranged between 38.6 and 49.5 ng/mL. A large pool of plasma was assayed in six replicates (mean 50.5 ng/mL) and was used to prepared medium (200 ng/mL + endogenous level) and high (4000 ng/mL + endogenous level) QCs.
Conclusion
- A HILIC LC-MS/MS method capable of assaying ManNAc in human plasma was developed.
- As an endogenous compound, the calibration standards were prepared in surrogate matrix, while the QCs were prepared in surrogate matrix as well as human plasma.
- The method was demonstrated to be accurate, specific for ManNAc in human Plasma and reproducible.
- The assay has been successfully validated to support clinical trials of ManNAc in GNE myopathy patients.
Acknowledgements
Yifan Shi, Meng Fang, Michael Zhang, Yinghe Li, Amy Wang, Ed Kerns, Nuria Carrillo-Carrasco, Xin Xu, Selwyn Yorke, Bradley Gillespie
Alliance Pharma, Malvern, PA; TRND, NCATS, NIH, Rockville, MD; New Zealand Pharmaceuticals, Palmerston North, New Zealand
Leidos Biomedical Research Inc. (formerly SAIC-Frederick, Inc.), Frederick National Laboratory for Cancer Research, Frederick, MD







