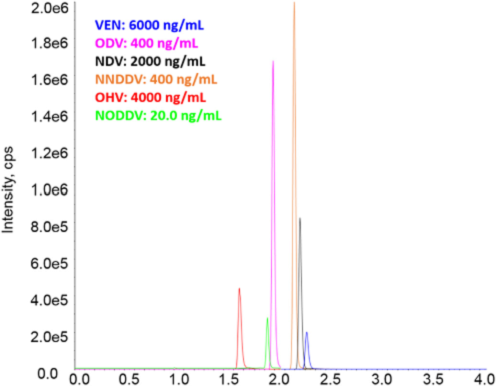
Our advanced scientific infrastructure and world-class experts attract trials from across the globe and meet their needs with the highest standards and efficient regulatory requirements.
Explore the extensive benefits of Australia’s clinical research ecosystem below.
- GLOBALLY ACCEPTED DATA
Alliance Pharma has a world-class reputation for quality data, following the highest level of GCP and ICH standards. Data from clinical trials conducted in Australia is widely accepted by all regulatory authorities including FDA and EMA. - EXTEND YOUR BUDGET BY 43.5%
includes bioanalytics services
almost halve your clinical trial costs
You can claim R&D cash refund with the Australian Government clinical trial rebate program. As an example, if you spend $200k on eligible R&D, you can receive $87k (43.5%) back from the Australian Tax Office (ATO). This effectively means you could turn $1million dollars into $1.7 million dollars of research spend in just three years. - RAPID START-UP & APPROVAL
The Australian Regulatory Framework is the fastest in the world for undertaking early phase clinical research trials
Trials can start in 4-8 weeks compared to 10 months in the US.
No IND required for clinical trials. Save up to a year in regulatory timelines and considerable costs.
- INDUSTRY-LEADING TECHNOLOGY
Clinical trials are resource-intensive processes requiring the availability of state-of-the-art equipment and facilities for testing and analysis. We have invested heavily in the industry-leading technology platforms to support speed, communications, and regulatory compliance. - GLOBAL CAPABILITIES
Local relationships, global execution As a global contract research organization – with sites in USA and UK – we can provide internal assay transfer and validation protocols among our global sites.
Harmonized SOPs, policies, and IT systems to facilitate transfer of methods, data, and information enable you to reduce time, risk, and cost. - SEASONAL DIFFERENCES
Seasonal differences to Europe and US, and time differences (our Australia lab works while you sleep)Seasonal differences between Australia and Europe or the US allow for yearlong participant recruitment for trials involving seasonal illnesses such as flu or allergies and allows researchers to cover seasonal variations in patient recruitment. - HEALTHCARE ENVIRONMENT
Australian clinical practices and some aspects of its health care system are similar to the United States, United Kingdom and most of Europe. - DIVERSE PARTICIPANT RECRUITMENT
The willingness of potential participants and their knowledge of clinical trials is another attractive option for sponsors to conduct trials in Australia.
A mixed population of Caucasians and Asian adds diversity to the participant pool and enables ethnicity differences in treatment response to be studied.
Need Support to Conduct Clinical Trials in Australia? Contact Alliance!





.jpg?width=2258&name=IMG_5349%20(002).jpg)
 Alliance Pharma has hired Dr. Ryan Klein as Director of Business Development. Dr. Klein has more than 20 years of experience in the pharmaceutical industry developing both oral and topical dosage forms for a range of indications. Dr. Klein began his career at GlaxoSmithKline, where he was an integral component of their drug discovery organization providing drug metabolism and pharmacokinetic expertise to project teams. He was instrumental in the development and implementation of a number of in vitro and in situ ADME models to assess drug absorption, metabolism, and disposition.
Alliance Pharma has hired Dr. Ryan Klein as Director of Business Development. Dr. Klein has more than 20 years of experience in the pharmaceutical industry developing both oral and topical dosage forms for a range of indications. Dr. Klein began his career at GlaxoSmithKline, where he was an integral component of their drug discovery organization providing drug metabolism and pharmacokinetic expertise to project teams. He was instrumental in the development and implementation of a number of in vitro and in situ ADME models to assess drug absorption, metabolism, and disposition.