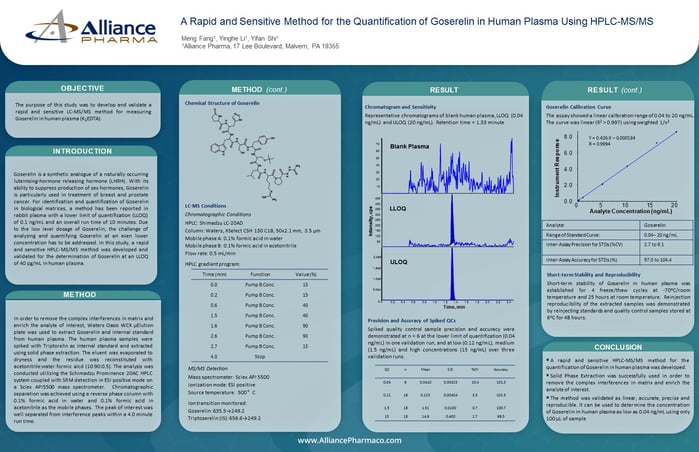
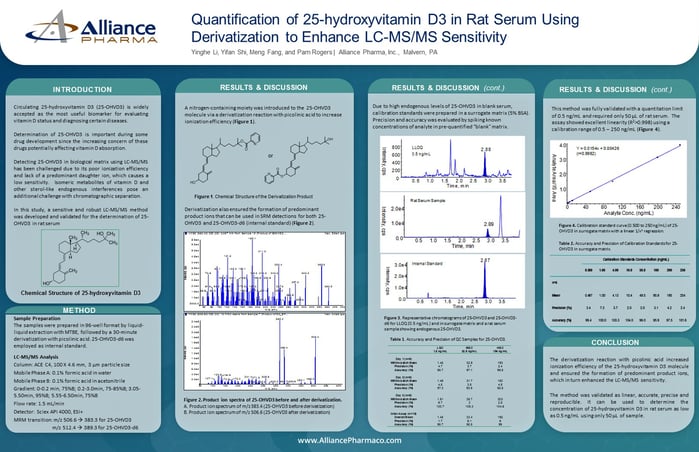
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) is a powerful technique in the field of bioanalysis, and it plays a critical role in drug development and clinical trials. This method has a well-earned reputation for high selectivity, sensitivity, and specificity for the detection and quantification of low levels of target analytes in complex matrices. The development and validation of a new LC-MS/MS bioassay is a complex and demanding process that involves assessing its performance via analytical characteristics. Even experienced scientists can face pitfalls when developing and validating a new bioassay, so to ensure that the LC-MS/MS method is reliable and accurate, it is essential to validate the method.
Here, we will summarize the eight essential characteristics of LC-MS/MS method validation, then explain why it is important that you work with an experienced contract research organization (CRO) to ensure quality in each of these areas:
- Accuracy
- Precision
- Specificity
- Quantification Limit
- Linearity
- Recovery
- Matrix Effect
- Stability
1. Accuracy
Accuracy refers to the difference between the measured value and the true value of the analyte. Scientists assess the accuracy of an LC-MS/MS method by comparing the measured concentration of the analyte in the sample to the known concentration of the analyte in a standard solution. Even small inaccuracies can lead to significant errors in the final concentration of the analyte, resulting in ill-informed decisions and, potentially, the risk of underdosing or overdosing a patient.
2. Precision
In LC-MS/MS method validation, precision refers to the degree of agreement between the results obtained through multiple measurements of the same sample under the same conditions, and it is assessed by calculating the variability of these results. Precise results are essential for reducing uncertainty in the final concentration of the analyte and ensuring the reproducibility of the method.
3. Specificity
Specificity refers to the ability of the method to accurately measure the target analyte in the presence of other sample components. This can be assessed by analyzing samples that contain the analyte of interest as well as other potentially interfering substances. Specificity is essential in LC-MS/MS method validation because it ensures that the method can accurately measure the analyte of interest without interference from other components in the sample.
4. Quantification Limit
The quantification limit is the lowest concentration of the analyte that can be reliably and accurately measured by the method. This is determined by analyzing samples with decreasing concentrations of the analyte until the signal-to-noise ratio (S:N) reaches a predefined level (20:1 to enable an increased chance it will be suitable). Defining the quantification limit is key because it provides an idea of what sort of sample extraction technique is needed, as well as determining the sensitivity of the method and the lowest concentration that can be reported. These are crucial to ensuring the accuracy of your results.
5. Linearity
Linearity is the ability of the method to produce results that are directly proportional to the analyte concentration over a defined range. In LC-MS/MS method validation, linearity is determined by analyzing samples with increasing concentrations of the analyte and plotting the response against the concentration. Linearity is essential because it ensures that the method can accurately measure a wide range of analyte concentrations.
6. Recovery
Recovery refers to the ability of the method to accurately measure the analyte in the sample after the sample has undergone extraction or other sample preparation procedures. Recovery is assessed by spiking the sample with a known amount of the analyte and comparing the measured value to the expected value. This process is essential because it determines the accuracy of the method for your specific sample matrix.
7. Matrix Effect
Matrix effect is the interference caused by the sample matrix on the ionization and detection of the analyte. Matrix effect is evaluated by comparing the response of the analyte in the sample matrix to its response in a pure solvent. The method should be able to accurately measure the analyte in the presence of the sample matrix without interference. This is essential because interference from the sample matrix or metabolites can impact the accuracy and precision of the method and cause variations in the analyte concentration. Careful validation is essential for optimizing methods that eliminate or minimize these risks.
8. Stability
Stability is the ability of the analyte to remain stable in the sample matrix under the conditions of storage and processing over time. It is evaluated by analyzing the samples at different time intervals and temperatures and comparing the results, across which the analyte should remain stable. Stability is essential to ensure that the method can provide accurate, reliable and consistent results.
The Key to Quality in All Eight Areas
Developing and validating a new bioassay using LC-MS/MS is a complex process that requires expensive instrumentation, advanced software, and, most importantly, tremendous expertise. Your CRO partner should have a team of scientists with the depth of expertise in LC-MS/MS method development and validation to ensure excellence in each of the eight essential categories listed above. To do this successfully, they should have access to state-of-the-art instrumentation and analytical techniques and be able to guarantee that the bioassay meets all regulatory requirements. That is the way to ensure that the method is carefully optimized and validated for your specific sample matrix, delivering the insights needed to make confident decisions for your program.
In the biopharmaceutical industry, LC-MS/MS assays require accurate, precise, and robust methods developed in the shortest time possible. At the UK lab of Resolian, we have successfully employed a protocol that reaches these goals consistently and efficiently. This achievement has enhanced our capabilities across the Resolian organization: Fordham and Sandwich (U.K.); Malvern, PA (USA); Brisbane (AUS).
Read the guide to discover our powerful approach to developing LC-MS/MS bioassays.