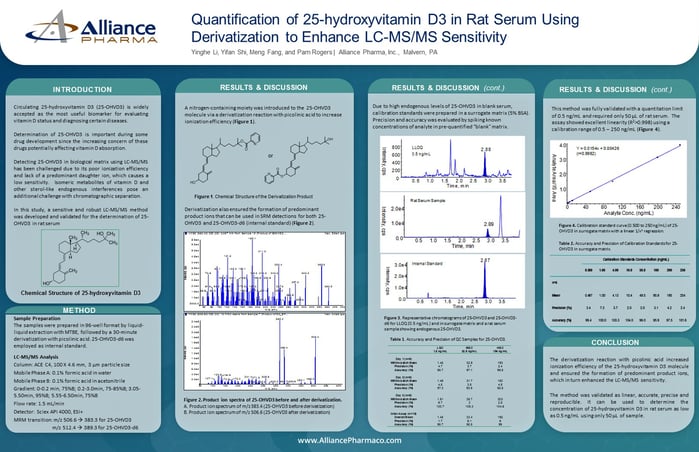
The purpose of this study was to develop and validate a LC-MS/MS method for assaying residual mercaptoethanol in in-process samples of a protein therapeutic product.
2-Mercaptoethanol is a widely used antioxidant in protein production and analysis. As a reducing agent, mercaptoethanol has the ability to cleave disulfide bonds, thus disrupting the tertiary and the quaternary structures of proteins. It is also included in enzyme solution to protect against catalytic site inactivation due to cysteine sulfhydryl oxidation or disulfide formation.
Mercaptoethanol is considered a toxicant, irritating the mucous membranes and respiratory tract, causing sore throat and abdominal pain, and potentially death if severe exposure occurs. As a result, determination of residual mercaptoethanol in biopharmaceutical products becomes crucial for patient safety.
Here, we report a LC-MS/MS method that utilizes derivatization with picolinic acid to quantitatively analyze residual mercaptoethanol and has been successfully used in biopharmaceutical manufacture.
Method
The abundant presence of thiols in protein biopharmaceutical molecules posts the major challenge in analyzing residual mercaptoethanol. We chose LC-MS/MS technique to measure the specific molecule instead of traditional methods which usually only detect the thiol functional group. By derivatizing mercaptoethanol with picolinic acid to form the corresponding dipicolinyl ester (DPE), a detection limit of 4 parts per billion (ppb) was achieved.
The samples were extracted with ethyl acetate, derivatized with picolinic acid, and then extracted with hexane again for analysis. Mercaptoethanol-d4 was used as the internal standard.
LC-MS Conditions
Chromatographic Conditions
HPLC: Shimadzu LC-20AD
Column: Agilent Zorbax SB-C18, 50 x 2.1 mm, 5 µm
Mobile phase A: 0.1% formic acid in water
Mobile phase B: Methanol
Flow rate: 0.6 mL/min
MS/MS Detection
Mass spectrometer: Sciex API 4000
Ionization mode: ESI positive
Source temperature: 500 °C
Ion transition monitored:
Mercaptoethanol-DPE: 289 → 166
Mercaptoethanol-d4-DPE: 293 → 170
Precision and Accuracy of Spiked QCs
Spiked quality control sample precision and accuracy were demonstrated at n = 6 at the limit of detection (4 ppb) in one validation run, and at low (10 ppb), medium (200 ppb) and high concentrations (320 ppb) over three validation runs.
Matrix Effect
Mercaptoethanol was spiked in the matrix as well as in water at n = 6. The matrix effect was calculated as the difference of the mean peak area of the spiked matrix samples and the mean peak area of the pure samples. The method had virtually no matrix effect observed (2.4%).
Derivatization
The low molecular weight of mercaptoethanol (78 amu) affected selectivity and required careful selection of SRM transitions. The presence of thiol and hydroxyl groups makes it difficult to use reversed-phase chromatography due to the lack of retention. Low organic content during gradient HPLC elution leads to poor ionization efficiency and sensitivity. To address these challenges, mercaptoethanol was derivatized with picolinic acid to form a dipicolinyl ester, which a limit of detection at 4 ppb was successfully achieved.
Conclusion
- A novel LC-MS/MS method capable of assaying residue mercaptoethanol in in-process samples of a protein therapeutic product was developed and validated.
- Matrix interferences or unknown impurity peaks are readily removed by two steps of liquid-liquid extraction.
- Derivatization with picolinic acid increases the assay sensitivity to reach a detection limit of 4 ppb.
- The method was demonstrated to be accurate, specific for 2-mercaptoethanol and reproducible.
Scientists
Yifan Shi, Yinghe Li, Meng Fang, William F. Wagner, Aston Liu, Sandro X. Nalli